world gathers for metal finishing
Q&As since 1989
-----
Extracting Silver From Photographic Fixer
Q. I have about 8 pounds of dry debris that came from a x-ray developer tank. The facility didn't change their fluid and only added to it. This debris I have had for 35 years. I am wondering if there is much silver present and if can be refined.
Will Wall- Miles City, Montana
April 17, 2022
⇩ Closely related postings, oldest first ⇩
Q. I am doing a chemistry project at sixth form college, and I decided to see if I could extract silver from photographic fixer. I have attempted experiments with help from my teacher, but with little success. I did manage it, but not very well. I still have further practical time, so I wondered if possible, if you could help me in any way. As I also need information of different techniques and equations, any information would be helpful.
Natalie Marks- Birmingham, England
2001
Refining furnace
on
Amazon
(affil links)
"Recovering Silver from
Photographic Materials"
by Eastman Kodak Co.
on
AbeBooks
or
Amazon
(affil links)
Q. Dear Natalie:
I am also working on the same project since last 6 months. However, I am presently having a problem of low efficiency.
- Mumbai, Maharashtra, India
2001
A. Hi Natalie,
You've worked on the project, but you tell us not a word about what you did or how you did it; then you say that you "did manage it, but not very well" -- but you don't give us even a tiny hint what that means. You said you got help from your teacher ... but surely your teacher recognizes that this is no way to do a science project! Please tell us what method you attempted, a few facts about how you set it up, and what results you got. I think people will try to help you, but guessing what you did and what results you got is silly.
Hi Virendra,
What do you mean by low efficiency? Are you applying substantially more electrical power than Faraday's Law suggests? Or adding substantially more reagent than stoichiometrically calculated? Or recovering substantially less silver than you think is in the solution? If the latter, are you sure how much silver is actually in that solution that you are trying to recover? The reason I ask is that we have hundreds of very similar questions on line here, with countless complaints that people are not getting as much silver as they expected. I find it less likely that no one has mastered the correct technique than that there is not as much silver in these solutions as people have been led to believe. Best of luck.
Please see also letters 12944 Consultation on Silver Extraction and 15559 Silver Extraction from Waste Hypo/fixer solution. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
May 8, 2010
Loss of Silver During Electrolysis?
2001Q. Silver Extraction from Photographic Waste Problem
Background - During the photographic film processing, Silver leaches into the fixer solution (sodium thiosulphate [on eBay or Amazon] ) from the photographic film and forms Silver Thiosulphate complex ions. The fixer is considered exhausted after becoming silver-rich (~ 7 gm/l), as it can no more serve the purpose of fixing the film in effective manner. Such exhausted fixer can then be processed to recover the Silver from it. There are various methods for this. I read about this business in newspaper and searched through the literature, internet, etc., and came to know the various techniques of Silver recovery from fixer.
I bought two 5 liter batches of fixer from a photography lab. and tried 2 methods with these two batches : 'Chemical Precipitation' as well as 'Electrolysis'. Estimated Silver content as shown by the Silver test strip (available in market) was ~ 6 gm/l (i.e. ~ 30 gm in each 5-liter volume). Considering loss during the recovery process, I was expecting at least 25 gm of Silver would be recovered.
Chemical Precipitation - As per literature, this process yields about 40-50% pure Silver. I added about 25 -30 gm of Sodium Sulfide (Na2S) in the 5 liter fixer solution slowly, until I reached the null point, i.e., when the Silver test strip showed absence of any Silver in the fixer solution. Then I filtered the precipitate (Silver Sulfide), which weighed about 25 gm. It was sent to a refiner. Refiner gave me 10 gm pure Silver. He charged heavily (Rs. 30/-) for this, as it was a small quantity to be refined. I incurred loss as the recovery was much less than expected (10 gm instead of 25 gm). 10 gm of pure silver could be recovered out of 25 gm of Silver sulfide, i.e. 40 % recovery, which is as expected for Chemical Precipitation technique (as noted in 1st Sentence of this para.). Hence, refiner might not have betrayed me, but the weight of the precipitate itself was much less than expected.
Electrolysis - As per literature, this process yields about 90-95 % pure Silver. I constructed an electrolytic cell in a plastic container out of two SS-316L electrodes (One cathode and one anode), an ammeter, a stirrer and two variable voltage supplies (one for stirrer and another for electrolysis). I passed a very small current of ~ 40 mA through the solution. (Limit on current was primarily due to the fear of Hydrogen evolution which would impair the process of Silver deposition on the cathode). Current decreased to about 30 mA after about 72 hours which I again restored to 40 mA by increasing the applied voltage. Over a period of about 90 hours, I got a very good deposition of Silver on the cathode. After that the silver test strip started showing 'No Silver'. Any further attempt of electrolysis was leading to deposition of black, inferior Silver Sulfide. Hence I stopped the electrolysis and scraped off the silver from the cathode. The silver flakes were weighed to be ~ 10.5 gm. After refining, refiner gave me 10 gm pure Silver. 10 gm of pure silver could be recovered out of 10.5 gm of Silver flakes, i.e. 95 % recovery, which is as expected for 'Electrolysis' technique (as noted in 1st Sentence of this para.). Hence, refiner must be genuine, but the weight of the deposit itself was much less than expected.
Conclusion - Thus, it can be seen that I got, in both the cases, ~ 10 gm of Silver as against the estimated ~ 25 gm. IS MY ESTIMATION PAPER FAKE? OR IS IT POSSIBLE THAT THE SUPPLIER IS ADDING SOME DYE IN THE FIXER TO DARKEN THE PAPER SO THAT HE WILL GET BETTER RETURNS ON HIS FIXER ? What is the solution to resolve this problem ?
VIRENDRA D [last name deleted for privacy by Editor]- Mumbai, Maharashtra, India
A. Hello Virendra. You have already demonstrated to yourself fairly conclusively that the refiner is treating you honestly, and that there is only about 10-11 grams of silver in each 5-liter batch, rather than the 25 grams that you expected.
My guess is that the test strips are inaccurate and the photo shop is using the same test strip to dilute the fixer to what he thinks is 5 gm/l before selling it. But we cannot see into the heart of the operator of the photo shop, and whether he is honestly trying to standardize to 5 gm/l or he is trying to leave much less silver in it knowing that the test strips will back him up. But our job here is not to try to catch potential cheaters, but to try to help with advise on technical subjects ... and the answer is that there is apparently only 10 to 11 grams of silver in the 5 liter batch, not 25 grams. Good luck.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
2001
Q. 1). Though the silver estimation paper shows the reported percentage of silver in the hypo solution, the silver extracted on the drum from the solution is less in quantity. Hence ever since I have purchased a machine, I am taking a loss and still have not made profit. The estimation paper show for example 4%, so that in 80 litres, I should recover 80 x 4 = 320 grams but I get only about 220-260 grams. So where is the other silver?
2). In some cases, where the solution is 3 months old, there is some white paste-like precipitation in that solution and in such solution not a single gram of silver gets extracted.
3). Some people have told me to pre-process the solution to concentrate it further and then process in the machine. But I do not know how to pre-process it and with what chemicals.
Further, if you have any information regarding extraction of silver from films (x-rays/photo negatives) kindly send the same.
Tikam Nihalani- Aurangabad, Maharashtra, India
A. Hi, Tikam. The 29-page booklet from Kodak, "Recovering Silver from Photographic Materials", may be helpful to you. And hopefully a reader will help you with your questions. But from numerous postings here, my first thought is that the silver estimation paper is inaccurate.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
June 2008
Q. Would you please answer this letter? Where can I find the answer. Thanks for finishing.com.
Dear sir, at the beginning let me thank you for your spirit of giving a hand of help to me.
I appreciate your assistance.
Here I demonstrate my construction idea with the diagram, so would you kindly identify the following points
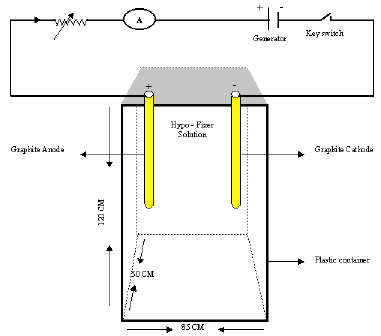
1 - should the electrodes touch the bottom of container.
2 - is there any specific graphite electrodes for this construction? If yes identify length and diameter .
3 - Does both length and diameter effect on extraction?
4 - what is suitable position of electrodes? Vertical or horizontal.
5 - How long is the distance between two electrodes?
6 - What is the type of current applied A.C or D.C
7 - What is the Applied Volts - Amperes - if I use Rheostat.
8 - Do the parameters Volt-Amperes, etc., remain unchanged if I use 5 liters of hypo instead of 30 liters?
9 - if I want electrolyte AgNO3 solution what about electrode are using - Ampere - Volt - Current ( D.C. / A.C. )
your faithfully
Maroky R [last name deleted for privacy by Editor]- Benghazi, LIBYA
A. Hi Maroky. I have not built such a device, so I don't really know the answers. Try your best to locate a copy of the Kodak booklet which experts wrote for you. Meanwhile, my educated guesses --
1. No need for the electrodes to touch the bottom.
2-5. I think it would be more practical if the cathode were stainless steel, and flat so you can easily peel the silver off; I suspect that the anode could be stainless steel as well. The electrodes should probably be flat vertical sheets facing each other, and spaced quite close together so power is not wasted to solution resistance, but not so close that a short circuit is possible when silver deposits on the cathode.
6-7. DC current is used. You want to plate out the silver at the lowest practical voltage and current because any electricity which is applied in excess of the amount necessary to reduce the silver ions will pull hydrogen from the water in the solution, changing it's pH and also leaving a spongy, burned, deposit. For a starting point, try 1-1/2 volts.
8. If you are doing a larger volume of solution, you would scale up the size of the tank and the anode & cathode size, retain the same anode-cathode spacing, same voltage, and same current density.
9. I have heard, but don't know for sure, that electrolyzing nitrate solutions releases toxic NOx gasses and should be avoided; I do know that nitrate solutions are never used for electroplating any metal at all.
Luck and Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. I want to know all about silver extraction from Hypo/Fixer &/ or photo waste. I am chemical engg. & do the hobby research about the silver extraction from hypo or photo waste.
Can somebody explain to me in detail about the construction & calculation of the equipment to carry out this process.
I have created small equipment with small transformer of halogen lamp & carbon electrode; is it correct approach?
Please do send me detailed illustrated reply as soon as possible.
Thanks,
chemical - Pune, Maharashtra, India
2006
"The Reducers Manual"
on
AbeBooks
or
Amazon
(affil links)
A. Hi Santosh, we appended your inquiry to a thread which offers some good hints. We very rarely see anyone provide a response which included drawings and calculations in this forum, but if you publish your detailed plans here, maybe someone like Maroky will respond with suggestions for improvements.
And please keep your eye out for a copy of Kodak's "Recovering Silver from Photographic Materials". Best of luck with your efforts!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
2006
2005
Q. I am engaged in the business of silver extraction from the waste hypo/fixer and waste photo films. At present even though I am extracting silver successfully my procedure is very lengthy, risky and slow process. I am adopting chemical treatment method. Is there any other easy and quick process for the purpose.
K. Sunitha- Hyderabad, India
2002
A. Hi Sunitha. Please describe your procedure as it will be helpful to the people waiting in line for answers in front of you while also allowing people to make suggestions. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
November 2013
Q. Dear sir
I am a radiographer working in one of the teaching hospital in Ghana and would know step by step method of constructing a silver extraction machine and also the method of extracting it, for I am a very good designer. We have a lot of this film and fixer solution which we have just been washing into the drain. May I know if I can set of a small company to extract this silver from this expired x-ray films and the waste fixer solutions very profitably?
Can I get a pure 100% silver?
Thank you
product designer - Accra-Ghana
February 19, 2008
Hi, Jamel.
It seems that the overwhelming majority of the people who post on that subject are dissatisfied with their recovery results: they are getting much less silver, making much less money, and working harder than they expected. My own explanation for such widespread disappointment is certainly not that all of these hundreds of people are too stupid to figure out good operating parameters -- but rather that someone out there is promising people more silver, more money, and less work than is realistic. That is to say, while the technology of silver recovery from photographic waste is very real, the promise of riches may be grossly exaggerated.
I would urge that people look in a library or spend a few dollars for the above-referenced booklet from Kodak, or a similar reliably scam-free instruction, before they spend many dollars on equipment and on waste silver solutions and then complain that it isn't generating as much silver as they hoped. I believe that no matter what they do it will not generate as much silver as they expected. Good luck.
Recovering the silver from the x-ray film is more complicated than recovering it from the fixer, so before you entertain the idea I strongly suggest that you google "film recovery systems" to appreciate how dangerous it can be. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
August 28, 2013
Q. I recently took over maintaining a dental hygiene school in a remote part of Oregon. The school changed to digital x-rays a few year ago before I got here.
The companies that pickup used X-ray fixer (silver nitrate, I believe) want a huge sum of money because we are in a remote location. The company that used to take it only came every few months when there was 30-40 gallons to pick up.
Now there is 5 gallons unused but past its expiration date. There is also a 5 gallon container with no lid and it has completely dried.
I have read on this site and would like to try and remove the silver from the silver nitrate.
My question is what and how do I go about the dried silver nitrate? Can I dissolve it with the unused silver nitrate? or is there a better way now that it is solid?
Also in both cases Solid and liquid. I would like to end up with something that does not need special disposal as that is difficult where I am.
Thanks!
Jon
dental hygiene - Oregon
A. Hi Jon. Accumulate all of your leftover stuff and call the disposal company one more time. You almost surely need permits to do this, which will cost you far more time and money for this, your final lot.
Two executives from Film Recovery Systems in Chicago made the cover of Newsweek years ago when they were convicted of first degree murder for the death (possibly by heart attack rather than cyanide poisoning) of one of their workers. I realize that you won't be working with cyanide or the actual film and am not trying to draw an exact parallel -- just warning that waste disposal is a very very serious business, not a sideline for a dental school to decide to engage in on a one-time basis.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
August 30, 2013
A. Hi Jon
Fixer for x-ray film is either sodium or ammonium thiosulphate.
The silver comes from fixing film and will be present as silver thiocyanate - not nitrate.
If the fixer is unused, there will be no silver content.

Geoff Smith
Hampshire, England
August 31, 2013
Q. 1) I want to know about the silver electrolysis method. How can we determine the production (pure silver) we'll get from our 550 liter one cell in per day which is powered by 20 Hz rectification unit?
2) what is the best conductivity of silver nitrate solution from which we produce good production.
3)
al kaloti factory - sharjah,sharjah,United arab emirates
July 26, 2016
A. Hi syed. I am sure you realize that such units do not "produce" silver, but instead recover some portion of the silver which is in the solution that you put into them. If the incoming concentration is high, they operate efficiently and can recover most of the silver. If the concentration is very low they may not be able to recover any of it. So what is the concentration of the silver-bearing solution that you are starting with? Importantly, how do you know? Where is the solution coming from (you added your question to a thread about extracting silver from photographic fixer, but I don't know for sure if used fixer is actually what you are putting into the recovery cell)?.
I don't know what you mean by 20 Hz rectification. There is surely a typo or misunderstanding there.
Your third question was blank, but please explain the whole situation, as that is probably the only way you can get useful responses. Thanks.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
July 2016
Q. What is the color of silver recovered from the photographic fixer bleach using electrolysis?
Abdulazeez adamu sulaimanStudent of Abubakar tafawa balewa university bauchi Nigeria - Bauchi, Nigeria
January 21, 2020
Q, A, or Comment on THIS thread -or- Start a NEW Thread
