
-----
Determine % Nitric Acid. Titration? Conversion factor?
Q. Hi Barb, I hope you're ok.
About finding the percentage of Nitric acid:
"%=1.33*v"
So 1.33 is a factor, but how come / where from?
Can you help?
- Tanzania
February 6, 2023
Ed. note: Barb's posting was from 8 years ago; we'll try to advise her of your posting but her e-mail and/or her situation has probably changed.
A. 1.334 is derived from below calculation:
% 42 Be nitric acid = (V of NaoH x (6.302/1.407/67%) )/ 5
Where 1.407 is wt/ ml of nitric acid 67 percent is the purity of 42 baume nitric acid and 6.302 is the equivalent factor of nitric acid
Sr. Chemist - Bangalore
March 3, 2025
Multiple threads merged: please forgive chronology errors and repetition 🙂
Q. If I have a solution of unknown Nitric Acid, how can I test that solution to determine that percentage of Nitric Acid?
Kimberly NeVilleaerospace - Ogden, Utah, United States
2006
|
A. Hi Kim, - Navarre, Florida A. Kimberly, - Auburn, Washington A. Titrate it against a known concentration of Sodium Hydroxide using a suitable indicator and calculate for the unknown. Compton, California, USA |
A. Jim, 8 is way too high a pH as metal hydroxides will form and not go back into solution in any reasonable time frame. Typically, pH's of 4 to 5 are used and have virtually no effect on the titration because pH is logarithmic.
James Watts- Navarre, Florida
![]() James - Thanks for the correction. Anyone in doubt: follow his statement, not mine.
James - Thanks for the correction. Anyone in doubt: follow his statement, not mine.
Sorry for the mis-information.
Compton, California, USA
A. If you want to check that which kind of acid is there you have given , then you please first confirm it acid by titration with caustic and find out the acidity. Then add barium chloride, silver nitrate, and lead acetate, in three different test tubes, and add that unknown and neutralized acid one by one. Check for any precipitation. If it gives white precipitates with barium chloride then that acid may be sulfuric acid, if it gives white precipitates with silver nitrate then that acid may be hydrochloric acid.
Rakesh [last name deleted for privacy by Editor]- Vadodara, Gujarat, India
Q. James,
The solution of Nitric Acid I am measuring is simply Nitric Acid diluted with water. I am looking for a volume percentage of the solution. We currently test the acid tank and report it in oz/gal. However, somebody decided that we need to report it in percent. The dissolved metals will be titanium and stainless steel.
- Ogden, Utah, USA
2006
A. Kim,
Simple if you have fluid oz per gal. Convert gals to fluid oz. Divide fl oz of acid by fluid oz of a gal and multiply by 100 to get %.
- Navarre, Florida
Q. We titrate our nitric acid bath using the following process:
- Pipette
[pipettes on
eBay or
Amazon [affil link]
5 ml sample into 250 mL flask
- dilute to 150 mL with distilled water
- add 2-3 drops of phenolphthalein
⇦ on
eBay &
Amazon [affil link]
Indicator (Gardotest indicator 2)
- gradually add 1.0N Sodium Hydroxide [1N NaOH on
Amazon [affil link]
(Gardotest Indicator 37) using burette while swirling until solution turns pink
- record the number of mL of 1.0N Sodium, Hydroxide used=V
- calculation is %=1.334xV
The discrepancy we have here is the interpretation of the end point ... different team members determine it at different points but we want to have a fail safe instruction/colour point. Our colour normally changes to a yellow-orange not pink as per above. Is the end point just when the clear solution changes to yellow-orange or you keep adding the Sodium Hydroxide until it is very dark and doesn't change anymore? Any advise would be appreciated.
aerospace - Orangeville, Ontario Canada
May 6, 2016
|
A. First things first, I'm not a chemist, but here's what I'd do:  Marc Green anodizer - Boise, Idaho A. I concur with Mr. Watts. pH 8 is too high. At that pH you're going to see a lot of NaOH uptake from the hydrolysis of transition metal ions.  Dave Wichern Consultant - The Bronx, New York A. Phenolphthalein is probably the worst indicator that you could use if you have any dissolved metal in it. You need an indicator that changes color in the 4-5 pH range. Methyl red
⇦ on
eBay &
Amazon [affil link]
and methyl orange
⇦ on
eBay or
Amazon [affil link]
are frequently used. Some like one better than the other. - Navarre, Florida |
A. Hi Barb,
Try bromocresol green
⇦ on
eBay &
Amazon [affil link]
; that gives a more defined end point than phenolphthalein, so there should be less room for discussion as to where the end point is.
Best Regards
Mark
Aerospace - Isle of Man British Isles
|
A. Bromocresol green ⇦ on eBay & Amazon [affil link] is a good indicator. Look it up and see what the color change points are. My foggy memory says that it had 2. If so, use the lower one. James Watts- Navarre, Florida A. The way I remember learning it, when you start to see the indicator changing color as the drop falls in and then immediately vanishing as it mixes, it's time to slow the drops way the heck down. Then, when the color changes all the way without reversing due to the mixing, that's the titration end point.  Ray Kremer Stellar Solutions, Inc. McHenry, Illinois  A. Good day Barb. Aerotek Mfg. Ltd. - Whitby, Ontario, Canada |
A. I agree that bromocresol green
⇦ on
eBay &
Amazon [affil link]
is the most user-friendly indicator for Nitric baths.
Personally I'm less of a fan of the methyl orange color shift, as it goes through quite a color range on its way to salmon red.
phenolphthalein definitely has its place in Sulfuric bath titration (and others). But as was alluded to earlier, by the time you hit the PTH endpoint, you're in the precipitation range for many metal hydroxides, and can easily get a false high reading if you're not prepared for them. In an anodize bath you take the hydroxides out BEFORE starting the titration (KF50% at a ratio of about 2 parts potassium fluoride
⇦ on
Amazon [affil link]
to 1 part bath aliquot); the difference between a treated and untreated sample is your aluminum value but that's another thread :)
You might notice that the pH endpoints are all vastly different, with MO around 4, BCG around 5, and PTH over 8... But once the acid is used up, the pH rises so rapidly that a minimal amount of titrant will cause it to skyrocket.
I agree also that it is an excellent habit to validate your titrations by creating a known standard and testing it. I did that with EVERY TANK when I took over my current lab. It's also a good instructional tool for new techs.

Rachel Mackintosh
lab rat - Greenfield, Vermont
Q. We titrate our nitric acid bath using the following process: (same as Barb Hulse above)
- pipette 5 ml sample into 250 mL flask
- dilute to 150 mL with distilled water
- add 2-3 drops of phenolphthalein
⇦ on
eBay &
Amazon [affil link]
Indicator
- gradually add 1.0N Sodium Hydroxide using burette while swirling until solution turns pink
- record the number of mL of 1.0N Sodium, Hydroxide used=V
- calculation is %=1.334xV
Does anyone have a reference for the calculation? We do the same thing and have been asked for a reference to support our results. I am also going to look into changing our indicator as they suggested. The Phth endpoint is really tricky.
Thanks for your help.
Aerospace - Niceville Florida USA
October 12, 2016
A. Hi Kate,
The indicator you should use is bromocresol green
⇦ on
eBay &
Amazon [affil link]
.
Also your multiplication factor looks like the one for sulfuric acid.
You should multiply your titre by 0.65. This works for a 10ml vat sample titrated with 1.0N sodium Hydroxide [1N NaOH on
Amazon [affil link]
, and give you a result expressed as percent by volume of 70% (700g/l) nitric acid. S.G. 1.41.
For 60% nitric acid use 0.76 as the factor.
I hope this helps, and good luck
Mark
making stuff for aeroplanes - A rain soaked rock in the irish sea
Q. We have been using a semi-automated titration system for our daily measurements. The system requires a daily pH calibration using 1.68, 4.01, 7.00, 10.01 and 12.45 standards.
We then transfer these values to an Excel spreadsheet for tracking the solution and making well informed solution adjustments. We have had great success with all of the titrations except for the Nitric Acid in solution of the DEOX chemistry.
Our DEOX target is 96 g/L of (Fisher Scientific Nitric Acid 65-70%)in solution
Our test process is:
Pipette a 2 mL sample
Add DI water up to 100 mL
Auto Titrate with sodium hydroxide
Press 'Start' Titrator will titrate to pH 3.7 endpoint. Results will be displayed as g/L nitric acid.
This nitric acid titration is very difficult to get a normal looking graph over time. The graph is very noisy.
I have conducted testing of the same process tank DEOX chemistry. Duration of all five samplings 30 minutes.
The first titration sample was extracted using a drum thief sampling the cross section of the tank without stirring the tank.
The result of the first titration was done using this sample.
Titration # TI00664 = 85.167 g/L
A second sample was taken and the extraction method was the same, no stirring and the drum thief was used.
Three consecutive titrations were run using this sample.
Titration # TI00665 = 88.088 g/L
Titration # TI00666 = 87.118 g/L
Titration # TI00667 = 87.533 g/L
The average of these three titrations was 87.579 g/L and % of deviation between the three samples was 0.5%
A third sample was taken and the extraction method was the same, no stirring and the drum thief was used.
One titration was done using this sample.
Titration # TI00668 = 86.601 g/L
My take away from this exercise was the three consecutive titrations of the same sample were showing repeatability of the semi-automated titration system.
The sampling method showed greater variation pointing to the chemisty not being homogeneous. I have since started stirring the DEOX tank using a stainless steel mixing paddle every morning. Then wait 15 minutes and take my sample using the drum thief.
I have not been tracking my results long enough but the graph looks less noisy.
I then spoke to the manufacturer in regards to this and I was pointed back to the manual titration using the bromocresol green
⇦ on
eBay &
Amazon [affil link]
indicator method.
I would like to understand the sampling methods of others out in the field. Should I go back to manual titrations?
What is the best method for day to day sampling? Use a dipper, drum thief or other method?
The first graph is of the proprietary chemistry in solution. It is easy to see how tracking and making adds is intuitive.
The second graph is what I was seeing when measuring the Nitric Acid.

Thank you All
Kurt Krueger- Lexington, Massachusetts
October 16, 2018
A. Hi again Kurt!
I read your post with a bit of a smile... You're going to drive yourself nuts over your DeOx! :)
Please don't! No doubt you've allowed yourself a pair of working ranges (target +/- some amount recommended by the manufacturer or your own experience, and then a narrower range like a warning limit) and your most simple, basic, goal for accuracy of the titration is to make sure that your titration error won't ever be enough to make you THINK you're in shop range when you're not, and that your adds will be accurate.
Your titrator sounds pretty repeatable especially considering the small aliquot being tested; I'm impressed. Usually smaller samples mean more analytical error, and 0.5% is outstanding.
Don't sweat the 'noise' TOO much in your samples over time. It happens. Reduction, rather than elimination, of the noise is about the best you can ask for. There's a lot going on, chemically, in a DeOx tank, and every new load of parts dropping off a new load of smut is going to slightly (even imperceptibly) shift how a free acid titration presents at that moment- It's larger trends over time that matter, and I totally get how you'd like to see your Nitric results looking more like your first graph. It's a chemical crockpot in there and you are correct that a reduction in noise may be achieved by ideal sampling.
To answer your question about sampling- you mentioned you swirl the tank with a paddle? Do you really not have a mixer in that tank (sparge, rotary, or recirculating)? If the tank is mixed well enough to function correctly, a dipper right from a few inches below the surface is absolutely fine. Rinse the dipper in the DeOx and dump it out a couple times before taking the sample.
The takeaway is don't get too crazy chasing perfection in a DeOx tank of all places, and you really want to make sure your tank is well mixed both at time of drawing test samples and time of use for best results with both!

Rachel Mackintosh
lab rat - Greenfield, Vermont
![]() Hello Rachel
Hello Rachel
Thank you for the quick response, You brought a smile to my face... You provide a valuable resource.
I was trying not to go nuts with the DEOX measurements, I wanted clean data. But yes, this can consume you.
I will be performing the bromocresol green
⇦ on
eBay &
Amazon [affil link]
titration to determine the nitric acid in solution and in parallel semi-automated nitric acid potentiometric titration. Until I have confidence in my process. Almost there.
I was not continually stirring this DEOX tank. I was only stirring this tank in the morning manually. That will be changed, I will be installing a stirrer. This may be the part I was missing...
Thank You
- Lexington, Massachusetts
October 17, 2018
Q. I am trying to test the nitric acid in my tank, what chemicals will I need with my titration kit to test the the wt percent in it with the copper, cobalt, iron, nickel and chromium.
Jevetta Siggerspickler - Hernando, Mississippi, America
April 26, 2019
A. Hi Jevetta. It's hard to offer good answers to a question until it is well understood. This pickling solution is straight nitric acid (no fluorides, sulphates, or other acids), and you would like a procedure for testing the wt percent of nitric acid ... but due to use it may be contaminated with those 5 metals? Or are you saying you want to know the procedures for measuring the nitric acid plus for each of those 5 metals? Is there a need for clarification or do you have questions or on any of the many comments already offered? Thanks!
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
A. Hi Jevetta,
For accurate quantification of metals, especially at low or low-ish levels, running by plasma spectrophotometry is standard. Test kits can give a crude result at higher levels but aren't particularly accurate, and are prone to matrix interference- you'd have to buy one specifically for a Nitric matrix. The good news is that Nitric acid is actually the required preservative for metals samples in aqueous solutions, and won't interfere at all with plasma spectrophotometry. You would do well to call around to a water testing or other analytical lab and find out what they charge- be sure to tell them what your matrix is- I can guarantee that it is MUCH cheaper than trying to buy all the chemicals, equipment, and training literature you'd need (not to mention labor costs!) to quantify various metals in solution. I've worked as a chemical analyst for well over a decade so far and, not to discourage anyone from learning how to do tests, it's time consuming and expensive.
AWWA

on eBay or Amazon
or AbeBooks
(affil link)
If you really want to know, grab a copy of "Standard Methods for the Examination of Water and Wastewater", being sure it's an edition that was written AT LEAST as recently as the 1990s ⇨

Rachel Mackintosh
lab rat - Greenfield, Vermont
Q. Is there any difference between using 1.0N or .5N sodium hydroxide ⇦ on eBay or Amazon [affil link] as your titrant? We have an auto titrator to test for nitric acid concentrations. We have always used around .75g of acid, filled our cup to about 40ml and then started the titrator using the .5N sodium hydroxide. We have a few customers who use 1.0N and all the methods on-line show 1.0N. I want to make sure using .5N is okay for what we are doing.
Stephanie Boll- North Bend, Ohio USA
October 28, 2019
A. Hi Stephanie,
If you use 0.5N on a titration that is calculated for 1.0, then logically you must then recalculate.
For example, if your titration used up 10.6 mL of 0.5N NaOH, it would have only used up 5.3 mL of 1.0N NaOH (because 1.0N is twice as strong as 0.5N) to reach the same endpoint.
Using a larger volume of a less concentrated titrant will increase resolution (though frankly plating-shop-floor titration is not a particularly precise branch of analytical chemistry lol) but you must remember to mathematically correct for any variation in titrant strength in your equations.

Rachel Mackintosh
lab rat - Greenfield, Vermont
Q. Hello everyone!
I've just built a production Passivation processing lab that'll serve the aerospace sector. I'm running Nitric Acid solutions in accordance with AMS2700, types 2,6, and 8.
In a recent audit, we received a finding for not having a titration method that is traceable to a national standard or chemical manufacturer.
I've been performing this process and the required solution analysis for 15+ years. In all those years, I've never been asked, "where did you get your titration method from?"
It's the method I was taught when I came into the field.
The quick version: with a stirring sample, I drop in 7 drops of Bromphenol Blue Indicator Solution, then burette in 1.00N Sodium Hydroxide to a change in color. The mL of Sodium Hydroxide used is multiplied by 6.45 equaling the Nitric acid concentration by volume.
Unfortunately, I'm not a chemist, and have no idea where this method derived from. I've found the 6.45 multiplier in the string, does anyone know where that number, or the whole method for that matter, came from?
I'd like to continue with this method, since I have all of the goodies to do it, but am more than willing to entertain a new method.
You all have been a great resource!
I appreciate it!
Thanks!
- Plainwell, Michigan
September 11, 2020
Tip: This forum was created to build camaraderie through sharing of tips, opinions, pics & personalities.
The operator & readers who are here for that often won't engage with anonymous posters.
A. Hi Josh L.
This is a nice question and there's an answer to it.
I also hope others will check the calculations and prove me wrong (or not)
You actually ask for an analytical chemistry course. I can recommend you A.Vogel's book about analytical chemistry ⇨
I didn't see how much solution you pipette, but that's not too important for the first part of the answer:
The titration you do is an acid-base titration, whereby the reaction is:
HNO3 + NaOH -> NaNO3 + H2O.
The theory in this titration is relatively simple. It is what we call an acid-base titration, whereby you neutralize the acid with the NaOH till the point that your indicator changes color, which is also the moment that all the acid is neutralized by the caustic (NaOH).
In this case you're lucky as the reaction equation goes like: 1 mole of HNO3 reacts with 1 mole NaOH to give 1 mole NaNO3 and 1 mole H2O.
(The mole is used in chemistry and is the unit of measurement for amount of substance in the International System of Units (SI). A mole of a substance or a mole of particles is defined as exactly 6.02214076x10^23 particles, which may be atoms, molecules, ions, or electrons)
Using the periodic chart you can find all the atomic weights of a mole of your elements and so find the grams of HNO3 that react with the grams of NaOH and you can even find how many grams of reaction products you get.....
Let's Look it up:
H = 1,008 gram per Mole
N = 14,01 " " "
O = 16.00 " " "
Na = 22,99" " "
So that means:
HNO3 + NaOH -> NaNO3 + H2O.
63.018g HNO3 + 39.998g NaOH --> 85g NaNO3 + 18.016g H2O
I must hope that the NaOH [1N NaOH on
Amazon [affil link]
you buy is from a certified supplier. That means that the amount of NaOH in the 1N solution is precisely weighted and dissolved to be exactly 1N. That way the whole titration is NIST traceable.
A 1N NaOH solution contains exactly 39.998 g/l. NaOH if you buy a certified solution and as long as you keep the bottle tightly closed, that is true.
However, you should keep in mind that, if you open up the bottle and use it for a long time, some of that NaOH will change to carbonate and your solution will not be exactly 1N anymore. Therefor, if you do this titration not very often, you can check your 1N NaOH, by buying also another burette and fill it with a solution of 1N HCl ⇦ on
Amazon [affil link]
.
If you now titrate a known volume of NaOH and use the same indicator and see if you need to titrate exactly the same amount of 1N HCl in ml to neutralize that NaOH solution (Indicator color change)you can check that NaOH standard in practice also.
If you find +/- 1% difference, I wouldn't be too concerned at all.
The next step is how you get to that 6.45 number and what it means.
I always prefer to express numbers in g/l or oz/gallons, as % HNO3 differs.
Let's assume that to increase your concentration, you add a solution of 50% HNO3 with a density of 1,310 g/l. (A well known industry standard)
As it is 50 mass %, it contains 655g/l. HNO3.
If I take a sample with a pipette of exactly 5 milliliter of this HNO3 solution, I have (655/1000)*5 = 3.275grams of HNO3 in that sample.
We have seen earlier that 1N NaOH contains 39.998g/liter
NaOH and that we can neutralize 63.018g HNO3 with that.
(HNO3 + NaOH -> NaNO3 + H2O.
63.018g HNO3 + 39.998g NaOH --> 85g NaNO3 + 18.016g H2O)
So this 5 ml sample that contains 3.275g HNO3 will need
(3.275/63.018)*39.998 = 2.0777g NaOH to be neutralized, or
(2.0777/39.998)*1000 = 51.95ml of a 1N NaOH solution.
As I don't know how much you pipette as a sample, I can't finish this for you, but I hope I gave you enough insight to do this yourself. If not, no problem, just ask.
Keep in mind that using "volume of HNO3" assumes that the HNO3 is always the same concentration, and I can guarantee you, it's not. Therefore, if your composition is critical, you better use the g/l. or oz/gallon calculation. If it is not, it doesn't matter too much, you probably have wide limits.
So to go back to your auditor's question: Due to the fact that you buy certified 1N NaOH standard solutions (not worth making them yourself, but you could if you had a good analytical balance and pure NaOH) and check them regularly with the 1N HCl solution, the outcome of this analysis is always guaranteed to be OK (as long as you have skilled people that do the titrations and you don't take shortcuts.) The latter you can test with an MSA.
Hope I didn't lose you. Sorry for my European units ...
Take Care,
Harry.

Harry van der Zanden
consultant - Tilburg, Netherlands
![]() Hello Harry.
Hello Harry.
First, thank you for your comprehensive reply! It is sincerely appreciated!!!
You didn't completely lose me, I did have to "chop a couple branches out of the way" to keep up...
That said, I will be purchasing a copy of A. Vogel's book.
I thought I would fill in a couple of the missing numbers from my original post. You are correct, I do purchase all of my standards from a certified source. They are reagent grade, and traceable to NIST.
1.) The volume of the working solution sample that I pipette for analysis is 1mL, then diluted to 100mL with Distilled Water for the titration.
2.) The concentrated HNO3 I use in the working solutions is 70%, reagent grade. (certified assay is 68.0-70.0 w/w%)
Specific Gravity: 1.42
Normality: 15.8
F.W.: 63.01
3.) The HNO3 % concentration limits for the 3 working solutions I use are: 20-25%, 25-45%, and 45-55%.
You've given immense insight. But, to be completely honest, I could really use the help filling in the blanks. I hope I've given enough additional information to do so. Please let me know if I've missed something.
Again, I appreciate your insights! Priceless!
Thank you!
Best regards,
- Plainwell, Michigan
October 8, 2020
A. Hi Josh,
Thanks for your kind words, that motivates to continue....
Recap:
The reaction in that titration is:
1 Mole HNO3 + 1 Mole NaOH -> 1 Mole NaNO3 + 1Mole H2O.
Or (the moles converted into grams, using the periodic table atomic mass data)
63.018g HNO3 + 39.998g NaOH --> 85g NaNO3 + 18.016g H2O
So a certified 1N NaOH [1N NaOH on
Amazon [affil link]
solution contains 39.998g NaOH
Your 70% (68 - 70), let's use 69% w/w HNO3 solution has a density of 1.42, which would mean 0.69*1420g/l. = 979.8g/l. HNO3
If you pipette 1ml and you need to find 6.45ml of 1N NaOH at the titration, than that 6.45ml of 1N NaOH neutralizes:
(6.45/1000)*63.018g HNO3 = 0.4065 gram of HNO3
Or that equals (as it is 1/1000th of a liter), a concentration of 406.5gram per liter HNO3 in your electrolyte.
Converting it into a volume % of 69% HNO3 would mean that the nominal composition = (406.5g/979.8g/l) = 41.5 volume % of a 69% HNO3 solution
If your target is 25 - 45%, you're good here.
But if your target is 45 - 55%, you're on the low side of the concentration.
In that case you would shoot for 50% by volume of a 69% (68-70) by weight nitric acid solution. (Density 1.42kg/l.)
As seen before, that 69% solution contains 69% of 1.42 = 979.8g/l. HNO3 and 50% by volume would contain 489.9g/l.
a 1 ml sample would contain 0.4899gram HNO3 if your solution were at exactly that 50%
Back to the general reaction:
63.018g HNO3 + 39.998g NaOH --> 85g NaNO3 + 18.016g H2O
That means that:
0.4899g HNO3 is neutralized by (0.4899/63.018)*39.998 = 0.31094g NaOH and as ml of 1N NaOH contains .039998g NaOH, you would need 7.77ml to be at the exact 50%.
In a similar way you can calculate your milliliters needed for a 20 - 25% v/v of the 70% solution.
One general advice, for those lower concentrated solutions (and actually also for the 50% one): you better pipette 3 ml of the solution. You don't want to be in a very low range with your burette, as your absolute fault in the reading remains the same over the whole burette range, but get's relatively pretty big if you titrate so little. Of course you need to change the factors too in that case.
Not being a passivation expert and it might be that people contradict me, but in general, the process window for passivating in HNO3 is pretty wide. +/- 20% concentration variation won't hurt, but you might not be able to start that discussion with an auditor without having your own evidence for that……
Good luck, hope this finally helps you out. Don't forget you've been doing this for 15 years an probably never had any complaint or problem, but alas, specification is specification and the AMS2700 is what you have to live up to I guess.
Take Care,
Harry

Harry van der Zanden
consultant - Tilburg, Netherlands
|
|
Q. HOW DO I CHECK STRENGTH OF NITRIC ACID AND GIVE ME FORMULA? MD SAJID- HYDERABAD & TELANGANA/WANAPARTHY, INDIA September 21, 2020
Q. How do you calculate the result of the experiment? OZDEMIR SEVİLAY- TÜRKİYE January 29, 2024 ? Hi cousins. More words please! This discussion may still be deficient, but we can't tell in quite what way :-( Thanks & Regards!  Ted Mooney, P.E. RET Striving to live Aloha finishing.com - Pine Beach, New Jersey Ted can be retained for immediate answers or long term project help |
Q. Hi Mark,
Can you explain where you got the 0.65 factor for 70% Nitric Acid?
Thanks!
- Fall River Massachusetts
October 6, 2021
A. Hi Casey,
The factor comes from a method in 'The Canning Handbook', page 935.
It is a well known old publication on electroplating.
It is no longer in print, but old copies can be found via eBay, etc. from time to time.
The factor is adjusted for a 10ml solution sample.
The 0.65 factor is used if you are topping up your tank with 70% wt/wt nitric acid, S.g. 1.41.
If you are topping up with 60% wt/wt nitric acid, S.G. 1.37, then the factor becomes 0.76.
I hope this helps.
best regards
Mark
- A foresaken rock in the irish Sea
A. Thanks Mark. The Canning Handbook is now apparently available as a paperback through Amazon (not vouching for the paperback publisher) or a Kindle e-book. They claim to be the 23rd edition, which was published in 1982 (my hardcover is 23rd edition).
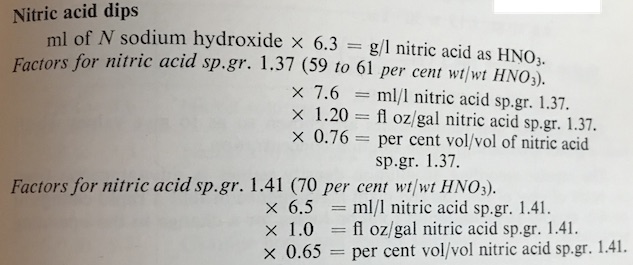
Readers: I read some reviews that said this is not a great plating book for a number of reasons including being outdated and relying heavily on proprietary names. My comment is that, indeed, it's not a good "first & only" book for those reasons and more ... but if you have a couple of others it's a really great one, offering practical approaches to so many issues :-)

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. Kurt,
Could you please provide more information on your semi-automated titrator setup? We are currently looking into getting one ourselves and have been having trouble finding the right balance between efficiency and cost. Any help is greatly appreciated.
Zander
- Denver Colorado
September 29, 2022
A. Good Afternoon Zander
We found the Hanna Instruments HI 902 semi-automated titration system (Now replaced by the HI932) to suit our needs. This system provided the accuracy, consistency between technicians(in comparison to a manual titrator results between technicians)and time to get results. All of the results are the saved and the results are transferred to a spreadsheet where we track/graph the titration results . We run this testing 3 times a week for the 5 separate titrations required for Chem Film process.
1.) Cleaner/Degreaser concentration in % by volume to water
2.) Deox grams/liter of nitric acid = (ml titration value)
3.) Deox grams/liter of Deox Concentrate = (ml titration value)
4.) Hexavalent Chrome Solution mg/ml
5.) pH of Hexavalent Chrome
All our Chem film titrations are completed in an average of one hour.
Hope this helps.
Cheers
- Lexington Massachusetts.
Q, A, or Comment on THIS thread -or- Start a NEW Thread

