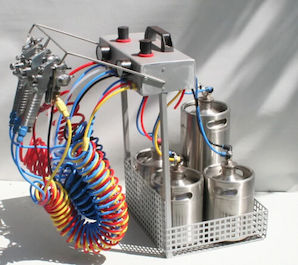
-----
Setting up a Simple Home Electroplating System
Quickstart:
Electroplating can be done at home by a dedicated hobbyist, but practice makes perfect and plating of precious parts shouldn't be attempted until some hands-on experience has been gained through plating of scrap. Large parts probably shouldn't be attempted until some experience has been gained on smaller ones.
Substrates that can be plated onto by a hobbyist include steel, brass, and copper. Most other substrates require specialized pretreatments, or are difficult for other reasons, and are a poorer starting point for people new to the art & science, trying to learn. Substrates which are poor starting points include aluminum
⇦ why?, stainless steel
⇦ why?, and zinc diecastings
⇦ why?.
Similarly, while copper, gold, nickel, or zinc plating is doable by a dedicated hobbyist, they should probably avoid silver
⇦ why?, brass
⇦ why? , and chrome
⇦ why? plating. "Spray chrome"
⇦ huh? is shiny paint; it contains no chrome and would be okay for hobbyists.
Be careful about selling plating services or plated parts, because you might be considered a plating jobshop rather than a hobbyist, and subject to a host of regulations. Read on ...
Q. HI, MY NAME IS RAY.
AS A HOBBY I RECONDITION OLDER MOTORCYCLES. I FREQUENTLY HAVE THE NEED TO CHROME PLATE OR NICKEL PLATE SOME COMPONENTS. I AM WONDERING IF IT IS FEASIBLE TO SET UP A PLATING SYSTEM IN MY GARAGE? I NEED TO KNOW WHAT A SIMPLE START-UP SYSTEM WOULD TAKE. I UNDERSTAND ONLY THE BASIC CONCEPT OF HOW ELECTROPLATING WORKS SO PLEASE BE SIMPLISTIC ON ME. HOPEFULLY WAITING!
RAYMOND J [last name deleted for privacy by Editor]HOLYOKE, Massachusetts
1999
A. Please see our FAQ "How Electroplating Works" and plate some scrap yourself -- it will cost nothing. Then see our "Introduction to Chrome Plating"; it may help you -- please give it a look!
Nickel plating as a hobby is one thing, but it's difficult for you to make a business out of plating in your garage due to OSHA safety regulations and EPA waste disposal regulations. And the toxicity of chrome plating solutions makes chrome plating in a garage completely impractical. I'd suggest that you focus on plating with nickel, optionally followed by gold plating.
The plating industry was the country's very first EPA-regulated industry, the trial balloon when the EPA was established, and the burden of compliance is heavy. Further, chrome plating solution is carcinogenic (think Erin Brokovich ⇨
and hexavalent chrome). Hobbies are one thing, but businesses are another, so if you charge a friend to plate something, you are starting on the slippery slope of being subject to reporting & disposal requirements. So please investigate the regs before buying chrome plating solution and becoming forever responsible for it.
Why do we say "forever responsible"? Picture what happened in the old days when a company generated toxic waste: they would hire a contractor for disposal. But sometimes that contractor turned out to be an evil midnight dumper who pocketed the disposal fees then dissolved the corporation and disappeared -- leaving the public to pay the cleanup costs. To address that problem the EPA came up with a simple rule: it doesn't matter who you ship hazardous waste to, or how much you paid to get rid of it, or how long ago it was: the "generator" of the waste remains responsible for it forever! Then add their 'joint & several liability' clause (which means if they can't easily separate your blobs of sludge from those generated by other people you're responsible for all of it) and you realize that this is a very bad thing for you :-(
So practice plating some small parts safely with kitchen chemicals, then move on to buying a quart or so of nickel plating solution ⇨
and practicing with it and learning. Best of luck, but be very careful to not buy chrome chemicals until you've learned a good bit!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
$29 to $80 kits chrome plating kits
Q. Funny, I have seen chrome plating kits in motorcycle mags. One long ago for 25 bucks. Recently for more but I can't remember where or how much. I want to plate the slides in Amal carbs. I am sure that this would improve their reliability. If you have any info on who would do this, please let me know.
p.r.k [last name deleted for privacy by Editor]- g'ville, Florida
2000
A. Hi PRK,
Yes, you saw plating kits for about $29 some years ago from J.C. Whitney -- today Eastwood offers them at around $75-$80, but these were/are zinc plating kits not chrome plating kits ⇨
Zinc is one metal, and chrome is something else entirely -- chrome is an intensely regulated carcinogen! If you wish to investigate entry level electroplating, you can contact a supplier of brush plating equipment and small systems like Gold Touch [a finishing.com supporting advertiser]. Or just googling "hobby plating supplier" will find them.
But if you spend some more time reviewing inquiries from earlier posters with similar interests to yours, you'll see why we urge caution and investigating the regulations before you buy the chemicals (in particular, chromic acid / chrome plating solution!) and become responsible for them. In America, once you use plating chemicals (probably once you open the bag), you're the "waste generator" and they become your waste forever! No matter how much you pay anyone to take, treat, or dispose of them, they are still your responsibility even 50 years from now & more. So look before you leap, and try some simpler stuff before considering chrome plating chemicals.
Good luck!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. Raymond J of Holyoke asked about a home plating system. Did anyone answer him ? If so what is the answer? I want to chrome plate my auto parts and will be 6' L x 18" W
James D [last name deleted for privacy by Editor]- Sylacauga, Alabama
2003
A. James -- brush plating and small tank plating of some items is clearly possible. But you seem to be speaking of an automobile bumper or some other very big copper-nickel-chrome plated item, and this would be a huge undertaking both in how much you'd need to learn to apply all the layers, and the effort of successfully plating such a large item. I think you may want to start much smaller and with a chrome-free plating solution. But read our Intro to Chrome Plating, and experiment with nickel plating of very small parts first..
Good luck!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Need Basic Plating Help
Q. Recently, I was given photocopied excerpt from a book supposedly published around 1890's. There were detailed instructions on how to perform nickel and silver plating. The instructions were; Use a 3-10% solution of Hydrochloric acid mixed with tap water, a 1.5 VDC battery and a glass container for the solution. Attach the source, nickel, silver, gold, to the positive side of the battery and the destination on the negative side and immerse them both into the solution.
Improvising, I have duplicated this process and have been able to successfully apply silver and nickel plating. I use a solution of baking soda [in bulk on
eBay
or
Amazon [affil link]
to neutralize the acid after the plating process. And I prepare the metal to be plated with acetone
⇦ on
eBay
or
Amazon
[affil link] Flammable!
.
However, after silver plating a piece of brass I noticed that it began to turn colors after a short time. And the only source I can find for nickel is a NS3 welding rod or a nickel coin. Which is 25% nickel and 75% copper. Everything that I nickel plate ends up copper colored until I buff out the finish. It then looks like chrome. I have varied the voltages and solutions to where I think I have the right combination. But I think I am missing something somewhere. Any ideas? Thanks.
- Longwood, Florida
A. One thing that 1890's book is probably missing, Patrick, is more than a century of progress :-)
Try to get your hands on a more modern book like the Electroplating Engineering Handbook ⇦ this on
eBay,
AbeBooks, or
Amazon [affil link]
.
You also need to know that in 1974 the EPA made electroplating the nation's first "categorically regulated" industry -- which means that even the rinse water, let alone the nickel and silver plating solutions, is a regulated material that you cannot discharge without treatment and a permit (please see EPA 40 CFR 430, which is available online). As long as you do not plate any item you sell, or plate anything for money, you probably remain a hobbyist, not a 40 CFR 430 business though.
For the nickel, the most important thing is you need to get proper nickel anodes⇨
Copper will deposit preferentially to nickel, and you'll be lucky to get any nickel to deposit at all if the plating solution is rich in copper.
Yours is probably not a very good nickel plating solution, but I guess it can work after a fashion for a hobbyist.
I'm surprised, however, that you were able to plate silver at all (and you might double-check that the deposit is indeed silver) since I thought all the silver would precipitate out of solution as silver chloride. Silver plating is not ideal for hobbyists: first, it's usually plated out of deadly cyanide solutions or proprietaries; second, you could generate a wildly explosive silver fulminate if you try miscellaneous additions; third, soluble silver salts are such a powerful biocide that casual neutralization with bicarbonate doesn't cut it environmentally.
As for the cleaning, please forget the acetone; use a tampico scrub brush
⇦ on
eBay or
Amazon [affil link] and wetted powdered pumice
⇦ on
eBay or
Amazon [affil link] , and a little strong detergent like Spic and Span
⇦ on
eBay or
Amazon [affil link]
.
Best of luck!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Brush plating vs. tank plating
by Kushner & Kushner

on eBay (rarely)
or Amazon (rarely)
(affil link)
Q. I am curious how well these home brush plating ⇦ huh? chroming kits. In particular, I have an old 1970's bicycle with chrome forks and rear stays, and chrome lugs. The chrome is almost intact, but there are lots of sand-sized rust specs in places.
If I can clean off the sand-sized rust specs, what will be the result of chroming using one of these home kits ($30 from JC Whitney, etc.) I am not looking for a perfect job, I'm looking for something that looks good at 5 feet and protects the finish so it doesn't rust anytime soon.
Donald G [last name deleted for privacy by Editor]- San Diego, California
2003
A. Hi Donald.
We're not trying to talk you out of a small investment in learning and experimentation. But quality plating with a real plating outfit is hard. Quality plating with a toy can be a joke. To chrome plate a bumper with a plating cell like the kits referred to would require thousands upon thousands of AA batteries. Your job is smaller, but look up Faraday's Law of Electrolysis and figure out how many dozens or hundreds of batteries you would need. And then you still have the issue of preparing the substrate so that your plating won't peel off, the fact that it's a chrome substitute not really chrome and it won't quite match, etc. It's a big job. We encourage you to try safe plating as a hobby, but not to start with the objective of repairing chrome plating.
Our site's focus is primarily industrial, where people typically spend thousands of times that much for a plating installation. Our concern isn't whether you spend $75 -- please do! And please try the plating experiments in our FAQ "How Plating Works", which will cost you nothing at all. Good luck! We just want you to start small and with relatively safe materials, not tempt you out onto a slippery slope that can pollute, be very costly, or possibly beset you with fines before you are ready. Good luck!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
|
|
- PORT CHARLOTTE, Florida December 27, 2023 A. Hi Patrick. Thanks for visiting & reading. But I see nothing wrong here! The OP is allowed to title his posting "How to Set up a Simple Home Electroplating System", others are allowed to tack their questions & comments on to it, I'm allowed to note that industrial plating setups easily cost way more than a thousand times the $75 of a hobbyist's starting point ... and you're allowed to help him with his questions or hold whatever opinions you wish :-) |
A. I use a home kit frequently to chrome aluminum pieces. Just have to treat it first -- remove the oxide layer and apply a layer of zinc -- I use a zincate solution.
MW Jansen- Southern California
2004
Ed. note: Glad you found it relatively easy, MW. We still suggest acquiring some experience plating onto steel or brass or copper before moving on to plating on aluminum.
Q. To MW Jansen: how about some details. I want to touch up a couple spots of chrome sheet metal.
Volts, amps, source of solution, what are anode and cathode materials, temperature, etc?
- Novato, California
2006
Dangers are overstated!
 This is just a comment, you can ignore it, but I don't think you will be able to based on the NUMEROUS posts in the past.
This is just a comment, you can ignore it, but I don't think you will be able to based on the NUMEROUS posts in the past.
This site DEFINITELY overstates dangers of home plating, I personally believe the reason is that if everyone found out how EASY it is to plate at home, the commercial shops would lose, LARGE...including yourselves.
I have been plating at home for years now, no problems. I find that there are dangers to EVERYTHING that a do-it-yourselfer must be careful of. Your attitude on this subject is "Don't get into woodworking at home, you could cut your hands of what with all those power tools." or "Don't get into painting at home what with all those fumes".
Honestly, RUBBISH!
How about doing us and yourself a favor and start posting educational responses to peoples questions and drop the 'tude.
Go ahead, blast me too. Oh yeah, I do take my waste to proper waste management facilities. Any "SHOP" can make mistakes as easily as a home do-it-yourselfer.
- Kingston, Ontario, Canada
2005
Jim: we're happy to print your opinion & everybody's -- that's what public forums are for! Here's all the space you want to tell people anything you want about what you've learned.
... but I didn't 'blast' anyone, and I don't own, manage, or work for a plating shop -- never did, never will -- so your claims of ulterior motive seem to paint you as a petulant adolescent.
This free site includes thousands of pages of information for students & hobbyists, plating book reviews, links to plating educational societies and training sessions, ASTM plating standards, free MIL standards, addresses of free plating libraries, and tens of thousands of highly detailed responses to plating questions & problems. We never censor postings (except ;comment spam' ads & ad-hominem remarks); please share your experiences instead of whining.
Still, although you don't seem to believe it, I do personally know two plating shop managers (one from PA, one from CT) who served penitentiary time for environmental crimes.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
 YES I AGREE PLEASE POST EDUCATIONAL RESPONSES ABOUT CHROME/GOLD PLATING JUST LIKE ANYTHING IT'S DANGEROUS..YOU AUTA SAY DON'T DRIVE A CAR YOU MIGHT GET IN A ACCIDENT...IF THE MARKET FOUND OUT ABOUT THE PROFITS THEY MAKE THEY WOULD LOOSE $$...I'VE BEEN PLATING GOLD FOR OVER 1 YEAR NOW WITH NO PROBLEMS NOW I'M EXPANDING INTO GOLD...WITH OR WITHOUT YOUR COMMENTS...FOR ANYONE INTERESTED IN CHROME OR GOLD PLATING YES YOU CAN DO IT IN HOME THEY ARE MAKING MORE AND MORE SYSTEMS FOR THE HOME OWNER...GOOD LUCK CAUSE I'VE HAD IT...AND IT'S NOT JUST ME!
YES I AGREE PLEASE POST EDUCATIONAL RESPONSES ABOUT CHROME/GOLD PLATING JUST LIKE ANYTHING IT'S DANGEROUS..YOU AUTA SAY DON'T DRIVE A CAR YOU MIGHT GET IN A ACCIDENT...IF THE MARKET FOUND OUT ABOUT THE PROFITS THEY MAKE THEY WOULD LOOSE $$...I'VE BEEN PLATING GOLD FOR OVER 1 YEAR NOW WITH NO PROBLEMS NOW I'M EXPANDING INTO GOLD...WITH OR WITHOUT YOUR COMMENTS...FOR ANYONE INTERESTED IN CHROME OR GOLD PLATING YES YOU CAN DO IT IN HOME THEY ARE MAKING MORE AND MORE SYSTEMS FOR THE HOME OWNER...GOOD LUCK CAUSE I'VE HAD IT...AND IT'S NOT JUST ME!
- Louisville, Kentucky
2005
by Reid & Goldie
-- hard to find & expensive; if you see a copy cheap, act fast!

on eBay or Amazon
or AbeBooks
(affil link)
A. Hi Tyrone: This isn't the Hotel California; if you've had it you can leave any time you like. But you're very welcome to tell people how to do electroplating at home. Here is all the space you want, an obviously eager audience, and it won't cost you a dime. So get to it -- unless your posting is just vacuous bitching.
Your analogy that our warnings are similar to telling people not to drive because they might get in an accident is perfect, thanks! The government requires that every driver be trained, tested, and licensed, and that every vehicle be registered, inspected, and insured. If you don't comply, you'll be fined, and in egregious cases you could even be jailed.
Similarly, the government demands training, testing, and licensing of all plating shops and their employees. Operate a plating shop without the registrations, the blood tests showing that the employees are safe, the discharge permits, the testing of bath surface tensions, the ampere-hour logs, the waste accumulation records, the manifesting, the annual hazwoper certification, or without advising your neighbors of the dangerous materials that you have on hand (Community Right-To-Know law), and you are subject to fines or even jail time. As I said, I know two plating shop managers who did hard time.
Hobbies are different than businesses and most of the regulations don't apply if it's just a hobby rather than a plating service. And you can probably operate a small plating business and stay below the radar, just as you can probably get away with driving without a license, registration, or insurance.
But in my town, as an example, we had a 'cancer cluster', so everything in the neighborhood was very deeply investigated by law enforcement; all neighbors were interviewed at length; names were published in books & court records. If a child in your neighborhood contracts cancer and her parents find out that you were doing chrome plating in your garage, discharging carcinogenic fumes into the air, God help you! It's not likely to happen of course; but if it does, your life as you knew it is over.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
![]() Chrome plating in your garage! Cover ups and conspiracies!
Chrome plating in your garage! Cover ups and conspiracies!
I was born into a custom chrome plating business. I worked there as a young teenager. I'm in my mid fifties now. Custom chrome plating is all I have ever done. I run the business. I know every aspect. I have done everything. Metal stripping, polishing, bead blasting, cleaning and activating for plating, cyanide copper plating, acid copper plating, nickel plating, chrome plating, customer relations, reporting to various agencies (EPA, DNR, local sewer districts, Etc.) All accounting and payroll. I know I am missing a few things, but I think Ted will understand.
And now Tyrone says it's a big cover up to deny people a chance to make millions in their garage. Oh Lord! Maybe we need a Canadian disposal place that doesn't require permits and testing as inferred in an earlier comment. To think all the years wasted when I could have done it in my garage and made millions!
Frank DeGuire- St. Louis, Missouri, USA
Q. Could someone please tell me a very inexpensive and simple way to practice EP (electroplating) using stuff I can easily buy or find in the home? The simpler the better because I'm on break from college and I need something to do. I became interested in this when I saw that a low charge battery could electroplate a coin in gold, but I have no idea what chemicals I need or how to do anything. Please help, I think this will be interesting and fun. I am a big guitar buff, and once I learn how to do this properly, I will likely EP my Floyd-Rose bridge if at all possible, or even EP a new set of strings to make them look cool. Thanks for our time,
Nick
student, hobbyist - Otego, New York
2006
A. Have you ever considered taking some courses on electroplating? The AESF (enter as a keyword on your PC) has beginner courses you can take on a correspondence level. Gold plating can be done at home, but I don't advise it. Gold metal plating salts are cyanide based, and you should be experienced in the field and have a clear understanding of the dangers involved. You would not want these chemicals in your house for obvious reasons. There are some chemical solutions that can simulate gold, but you don't want them in your house either. Does your college schedule allow you to work part time in a plating shop to gain experience? You want to stay away from stovetop electroplating, I've heard some horror stories. Good luck in college.
Mark Bakerprocess engineer - Malone, New York
A. There are some sheets in the FAQ section of this website that describe how to set up demonstration sets for electroplating - they would, for example, let you duplicate the plating of a coin with a battery.
However, to get a high quality and adherent coating requires the use of equipment and chemicals that are both expensive and dangerous; not something you'd really want to do at home.
If you want to try to do your guitar pieces anyway - just for the novelty of saying you did it yourself (and some people are able to get good results, depending on the process) - search through the archived threads here on finishing.com and you should be able to find enough info to make a passable attempt.
Good luck either way!
Compton, California, USA
A. Gold plating a coin, or an item that was previously nickel-chrome plated, with a brush plating machine is a quite practical project for a hobbyist because it's simply a matter of removing the chrome but leaving the underlying nickel plating in place, getting the item clean and active, then gold plating it. Projects that require mechanical prep are more difficult.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. Hi to those of you who will answer questions and not whine. I was wanting to replate the pot metal parts from my 68 El Camino. What's the best way to prep the parts with moderate pitting, and should they be treated as an aluminum part in the plating process? Last but not least if you're not willing to eat it or drink it, then treat it as a nasty substance be responsible. Dumping it down the drain goes right back into your drinking water. Thanks.
Michael Waltersnovice - Water Gap, Pennsylvania
2007
A. Hi, Michael. "Potmetal" may be zinc diecastings or aluminum diecastings (they look just about identical although aluminum is much lighter). But a 1968 car probably used zinc diecastings rather than aluminum, so zincating probably isn't necessary.
It isn't easy to fix those pits because the porosity absorbs water and plating solutions, thus causing contamination; plus the absorbed water or gases can come back as steam when the parts are heated and that causes holes and blisters. The usual way to deal with mildly pitted diecastings for restoration is to copper plate them in cyanide copper plating solution and "mush buff"; this means basically to "mush" the soft copper plating into those pits by/while buffing. After the pits are pretty much filled with copper you can go on to more copper plating, then nickel plating, then chrome plating. Deeply pitted castings, however, require a plating artist to hand-drill & hand-fill every pit, so each casting will cost hundreds of dollars to plate.
Good luck!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. Could I electroplate with a AAA (1.5 volts)?
Joe Wilsonhobbyist - Virginia Beach, Virginia
2007
A. For the purposes of a school science project, yes you can, Joe! Please see our FAQ: "How Electroplating Works".
For practical electroplating probably not, though. Look into Faraday's Law because plating is energy intensive and that AAA battery is only going to be able to electroplate a very thin layer of metal on an item the size of a dime before it's exhausted.
To understand why, recognize that a battery and a plating cell are, at least in principal, actually the same thing running in opposite directions. In electroplating we use a bigger battery or power supply to overpower the battery formed by the plating cell and force it to run in reverse. Your AAA battery isn't going to win this battle of the batteries except when plating a very small part.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. I have no experience with plating, but I have a great deal of experience with OSHA and EPA. The thing that would scare me the most about plating in my garage and disposing of the hazardous waste is this. Even if I did all the paperwork it requires to order, store and use the materials, then to dispose of the materials as hazardous waste, the thing that would not let me sleep another night I lived is the "Cradle to Grave" rule by EPA. Once you dispose of the material, the paperwork has to be maintained forever! And should the container you dispose of this material in should leak, then you the disposer are responsible and legally liable. The cost of a cleanup could be millions, and the fine is $25K per day until it is cleaned up. And you have to pay to reseal the junk, restore the junk and that means everyone else's junk stored with it. I just want to know, since I am just starting, what a fair price to pay for plating. I am restoring an old car and I want to plate the bumpers, etc. What would be a fair price?
Gus Weaverhobbyist - Harrodsburg, Kentucky
2007
A. Hi, Gus. The biggest cost of most things including plating, especially replating old stuff, is labor. It would be fair for a plating shop to charge you about the same amount for their time as a plumber or mechanic would -- maybe just a little more because of the cost of material and because a plating shop's equipment costs more than a plumber's equipment.
So the real issue is how long will it take, and this will depend on the condition (how much buffing and polishing is required) and on how high quality the job is. Reworking a single old bumper involves far more labor hours than manufacturing from scratch a new mass-produced one. So, unfortunately, it usually costs at least the same as a new bumper, often more; and replating an old diecast hood ornament, with all the attendant drilling and filling of pits, can easily cost 10x-20x the cost of a new one.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
A. Ted is on the money with everything he has said. I began looking around for a home based chrome plating kit...thinking it was that easy. It costs about $500 here to strip, repair and rechrome a vehicle bumper. In my wisdom, I thought I could do it for next to nothing. I came up against ALL the hurdles Ted has mentioned. I paid the $500 and have a fantastic bumper and no headaches. I am impressed with your site and knowledge Ted...keep up the good work mate....Peter
Peter Carey- Perth, Western Australia
January 11, 2008
![]() Thank you the kind words, Peter!
Thank you the kind words, Peter!
A quick aside -- some people love working on their boats -- a beer in one hand, scraper in the other. I ask on boating forums where to get something done, and they bend my ear about how I can do it myself. They don't comprehend that some of us hate this stuff, and our only interest is in getting someone to do it.
Maybe part of the "friction" here is that someone will say they want something plated and I read into it that they just want it done. Meanwhile, enthusiasts who enjoy hobby plating believe that the person would love to electroplate it themselves and we're stomping on their joy :-)
I especially appreciate your posting because it implies that you didn't particularly like the idea of electroplating yourself -- you only tried it to try to save money.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. I would like information on how one would go about starting a chrome plating business on a small scale.
Any information would be helpful.
- Cherryvale, Kansas
April 8, 2008
A. Hi, Maynard. I think you'll find our previously mentioned Introduction to Chrome Plating will give you a quick but good feel for what the chrome plating business is about.
I would strongly urge people to try their best to work a summer in a plating shop before volunteering to become forever responsible for the toxic chrome chemicals they will need to buy.
If that isn't possible, at least join the National Association of Surface Finishers (nasf.org) and attend some local monthly meetings and the annual Sur/Fin convention, take a 2 to 4-day introduction to plating through NASF or Kushner Electroplating School, browse through a few of the most important books. Good luck!
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Chrome-look paint, aka "Spray chrome"
A. Hi I can give you a good answer to this. I purchased some paint called Mirrorchrome. I have never in my life seen a chrome paint that actually shines like chrome until now. It took me several tries but finally after the fifth one I figured it out.
You prep your item just as you would for any paint job then spray on a Black gloss (I used an over-the-counter Base clear black). Clear coat it, then comes the tough part, wet sand it all the way to 4000 grit then polish it to a mirror shine then just spray on the chrome. It takes about 15-20 min to flash over then polish it with a lint free cloth then let it cure for an hour then spray a coat of the clear over it. I did find you have to use a base clear clearcoat all others put a haze over the chrome. Anyway check out Alsa and see their videos; it does work.
Scott Lancaster- Norridgewock, Maine
August 10, 2008
![]() Hi, Scott. Yes, chrome-look paint is, for the amateur and hobbyist, a great alternative to real chrome plating. Thank you for providing detail on what worked for you!
Hi, Scott. Yes, chrome-look paint is, for the amateur and hobbyist, a great alternative to real chrome plating. Thank you for providing detail on what worked for you!
There are many suppliers of "chrome-look paint" systems, and we try to discuss technologies rather than bringing specific company names into it (huh? why?).
Thanks again!
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. Can I do some Chrome and Brass plating at home?
I am restoring a 43 ft. 1958 boat. Lots of bright work to be cleaned up and some of the parts are hard or impossible to replace. I have sent some things out for professional plating, but the cost can be daunting. I am a chemical engineer, so I appreciate the environmental and other operating costs of the professional shops. I was just wondering if there are any replating kits and instructions that I might use safely at home to do some of the small plating work. Thanks for you help.
Bill Relihanhobbyist, conservator - Apollo Beach, Florida
March 19, 2009
A. Hi, Bill. As you see, we appended your letter to a similar thread. We have an "Introduction to Chrome Plating" on line here that will help you understand what is involved. I would not suggest real chrome plating at home because it requires toxic and carcinogenic hexavalent chromium, but there are imitations available. The best brass plating solutions are cyanide based, which is an extremely powerful poison, definitely not for home, but you may be able to find a non-cyanide brass plating solution (from EPI / Electrochemical Products Inc. [a finishing.com supporting advertiser], for example, or a hobby plating site, that is good enough.
We've added a summary to the beginning of the thread which explains why copper, gold, nickel, or zinc plating are more practical for amateurs than brass or chrome plating.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. Hi my name is matt, I am very much into building cars and motorcycles and just about anything custom. I have never had anything plated and I understand the prices can be pretty high, I am just wondering if there is any type of metal plating I can do at home in my garage for my self, I actually have a set of wheels that are hard to find and would like to try to plate my self, (the chrome is peeling on them) I would also like to plate a lot of other parts under the hood of the Camaro I am building, it's a 1979 Z28 and I am trying to completely restore it, and there is a lot of things I would love to chrome plate and don't want it to cost me a arm and a leg. I don't mind to labor at all I found a kit for 899.00, this is close to the kit I found, the difference is the kit I found is 6 gallon instead of 4.5, I would like to know also how much plating will a 6 gallon kit make and do you have to have any kind of license or permit to buy the kit. I understand it is an art but I do like learning and would really love to learn the trade without spending over a 1000.00 dollars, I would also like to know if there is any kind of newer technology that would make it safer and easier for someone with no experience and the finish I am looking for with the automotive parts. I have always been a quick learner and believe I can do it. There are also a lot of books that say how easy it is to do it at home and I don't believe that and am kind of sketchy about them. Any help would be greatly appreciated, thanks. P.S.: this is the kit I found, like I said it's exactly the same except bigger 6 gallon not 4.5 Gallon Kit
6 x 6 Gal Plating tanks with lids
2 x 6" x 8" Nickel anodes & bandages
2 x 4" x 8 Copper anodes & bandages
2 x 12" x 12" Chrome Anodes
2 x 8" x 8" GP Plates
3 Pack Nickel Crystals w/Brightener (Makes 4.5 Gals)
3 Pack Copper Crystals (Makes 4.5 Gals)
10 x 1.5 oz Copper Brightener A
3 x 4 oz Copper Brightener Part B
3 Cans Chrome Crystals (Makes 4.5 Gallons)
Chrome Activator
1 x 4.5 gal Flash Copper Chemicals (A, B, C)
2 Packs SP Degreaser (Makes 8 Gallons)
4 x 300 W Ceramic Heaters
1 x 200 W Glass Heater
2 x Thermostats
3 x Filter/pumps (For Nickel/Copper & Flash Copper Kit)
Fume Control Balls
2 Pack Pickle #4 (Makes 4 Gals)
Manual and DVD
beginner - Greeley, Colorado
March 17, 2009
A. Hi, Matt. We appended your inquiry to an earlier thread so you can conveniently read a number of different perspectives, and we hope you'll get further responses.
I personally would not buy even a small plating system until I had first done simple plating with kitchen chemicals, and followed it up with some nickel plating from a quart or so of solution and a bucket ... and wouldn't even think about buying chromic acid until truly confident after substantial experience with plating (if ever).
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
(You're on the 1st page of this topic) Next page >
Q, A, or Comment on THIS thread -or- Start a NEW Thread
 on eBay
on eBay